Biosimilars
What are biosimilars?
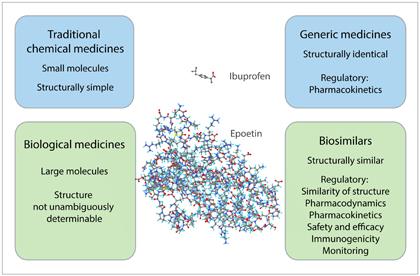
A biosimilar medicine, or biosimilar, is a medicine which is similar and comparable to the biological reference medicine. Biosimilars are not directly comparable with generic medicines, in which the chemical composition of the active substance is identical to that of the reference medicine.
Biosimilars contain the same active substance as the reference medicine, but a different version of it. Some differences may occur due to the manufacturing process and the complexity of the molecule structure. With biosimilars, as with reference medicines, there may be some natural variation between different batches.
When applying for marketing authorisation, the manufacturer must be able to prove that this internal variation and any differences between the biosimilar and the biological reference medicine do not affect the safety or efficacy of the medicine.
Generally speaking, biosimilars can be used for the same indication as the reference medicine. Similarly, any limitations applicable to the reference medicine also apply to the biosimilars.
Why do we need biosimilars?
Biological medicines are, as a rule, very expensive. While the high cost can be partly attributed to the demanding manufacturing method, high development costs and significant therapeutic value, lack of price competition is a major contributor to the high price. The idea behind the development of biosimilars is to increase competition and provide more therapeutic options by reducing the regulatory requirements associated with marketing authorisation, thereby enabling the development of biological medicines similar to the reference medicine.
However, as the manufacture of biosimilars is also very challenging and expensive, the prices of biosimilars are likely to remain relatively high. It is unlikely that competition in biosimilars will be equal to competition in generic medicines after the expiry of patent and data protection rights.
Despite such limitations, the prices of biosimilars are expected to be lower than the reference medicines. It is also likely that competition will lower the prices of the reference medicines.
Read more
- Biological medicinal products
- Fimea's position
- List of available biosimilars in Finland
- Uptake of biosimilars in Finland - Physicians' views
- Automatic substitution of biological medicines at pharmacies - Views on potential automatic substitution and the related medication safety aspects
- Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU