Information for medicine users
A biological medicine can be substituted at the pharmacy for a cheaper alternative. In drug substitution, the cheapest interchangeable, i.e., similar medicine is offered, which can be the original preparation or its biosimilar. Biosimilars and the original biological medicines are equally effective and safe. Drug substitution increases price competition and curbs societal drug reimbursement costs.
Since 2017, doctors have been obliged to prescribe the cheapest biological medicine suitable for the patient's treatment. Prescriptions for biological medicines are valid for only one year at a time.
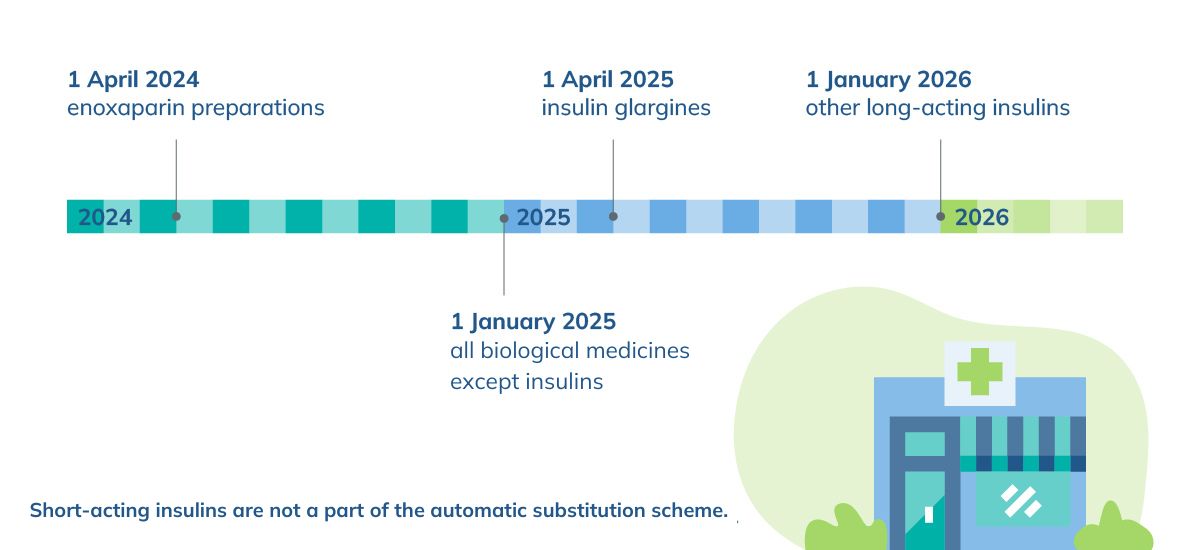
Drug substitution started gradually in pharmacies in April 2024. It began with enoxaparin preparations, and the last included in drug substitution will be long-acting insulins in January 2026. Short-acting insulins are not subject to drug substitution. Biological medicines for patients under 18 years old are not substituted at the pharmacy.