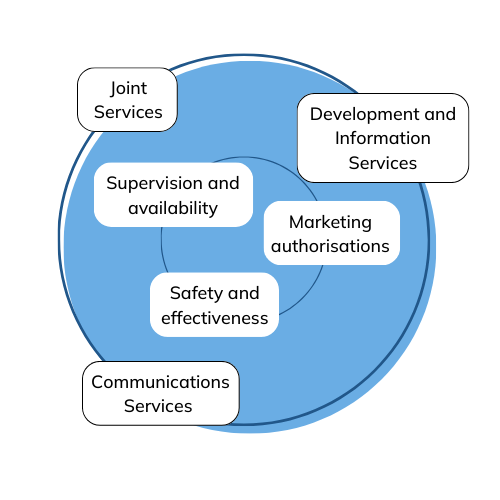
We develop the management of Fimea and its human resources and take care of Fimea's strategic development.
We take care of HR management, the pay system and activities related to collective bargaining at Fimea and manage Fimea’s agency and support services.
We are responsible for the development of the work environment, the coordination of safety and risk management, and the management and support of procurement.
We manage financial planning, the budget, the distribution of operating appropriations, the agency’s accounting and its duties as an accounting unit, and the agency’s payment matters.
We manage Fimea’s legal matters, provide legal advice and represent Fimea in courts.
We maintain, plan and develop information management, document management and archive operations at Fimea.
We prepare and coordinate sustainability reporting and maintain and develop the quality system applying to the entire agency.