Medicines that have marketing authorisation in Finland
The marketing authorisation system ensures the efficacy, safety and quality of medicines on the market.
The marketing authorisation system safeguards medicinal product users
Medicines are used to prevent or treat illnesses. Before a medicinal product can be sold to the consumer, the pharmaceutical authorities will grant it a marketing authorisation once they have verified its efficacy, safety and quality. Even after entering the market, the medicinal product is closely monitored and its product information is updated on the basis of the accumulated safety information throughout its life cycle.
A pharmaceutical company applies for marketing authorisation for a medicine
The decision to apply for marketing authorisation for a medicinal product is always made by the pharmaceutical company and the licenced companies responsible for importing medicinal products to Finland.
Pharmaceutical companies can apply for marketing authorisation for their products throughout the EU or for marketing authorisation in some individual countries.
The marketing authorisation procedure is based on the marketing authorisation applications submitted by pharmaceutical companies. The pharmaceutical company must demonstrate, by means of research results, that the benefits of the medicinal product outweigh the associated risks when the medicinal product is used for its intended purpose (treatment or prevention of disease), the target group and with the dosage and other conditions of use proposed by the pharmaceutical undertaking.
The application consists of clinical trials on the efficacy and safety of the phases (phases I-IV) of clinical trials in volunteers and pre-clinical documentation, i.e. pre-clinical trials on medicinal pharmacology under test tube conditions and possibly animal testing. The clinical trials mentioned above for a medicinal product already in use can sometimes also be replaced by reference to studies already performed on another similar medicinal product or published scientific literature. In addition, the pharmaceutical company must demonstrate through extensive tests that the medicinal product meets the product-specific requirements in terms of chemical and biological quality.
The pharmaceutical regulatory authority assesses the application and grants the medicinal product marketing authorisation when the benefits of the product are assessed as higher than the risks (a positive benefit-risk ratio).
Common procedures in place in the EU/EEA
As the Finnish marketing authorisation authority, Fimea operates in close cooperation as a member of the EU/EEA pharmaceutical supervision network.
In the EU/EEA, the marketing authorisation for a medicinal product can be either national marketing authorisation for a particular Member State, marketing authorisation for a mutual recognition or decentralised procedure for several countries simultaneously, or marketing authorisation for a centralised procedure for the entire EU territory. Centralised EU-wide marketing authorisation is granted by the European Commission at the proposal of the European Medicines Agency on the basis of an assessment carried out teams of experts from selected national pharmaceutical agencies.
Under EU pharmaceutical legislation, all new pharmaceuticals and biological medicines are referred for evaluation through the European Medicines Agency to a centralised procedure. In health emergencies, marketing authorisation may also be granted as conditional or by means of a rolling review.
Medicinal products for humans and animals are assessed separately in their own marketing authorisation processes.
Safety information accumulates throughout a product's time on the market
Monitoring of medicinal products will also continue while their marketing authorisation is valid. In particular, information on safety will grow more accurate as the medicine is used by large groups of patients. Changes to the marketing authorisation shall be made, if necessary, in accordance with this new information. If safety concerns arise in the use of the medicinal product, the terms of use may be restricted or other safety measures may be added.
The pharmaceutical authority is very rarely required to withdraw the marketing authorisation for safety reasons. The most common reason for cancelling a medicinal product's marketing authorisation is that the pharmaceutical company no longer considers the marketing of the medicinal product profitable. The authority regulating pharmaceuticals does not remove medicinal products that are in use from pharmacies on its own initiative.
A voluntary compensation system for pharmaceutical injuries is in force in Finland to protect pharmaceutical users: Finnish Mutual Insurance Company For Pharmaceutical Injury Indemnities. The insurance covers unexpected adverse effects caused to users by medicines sold in Finland or released for consumption.
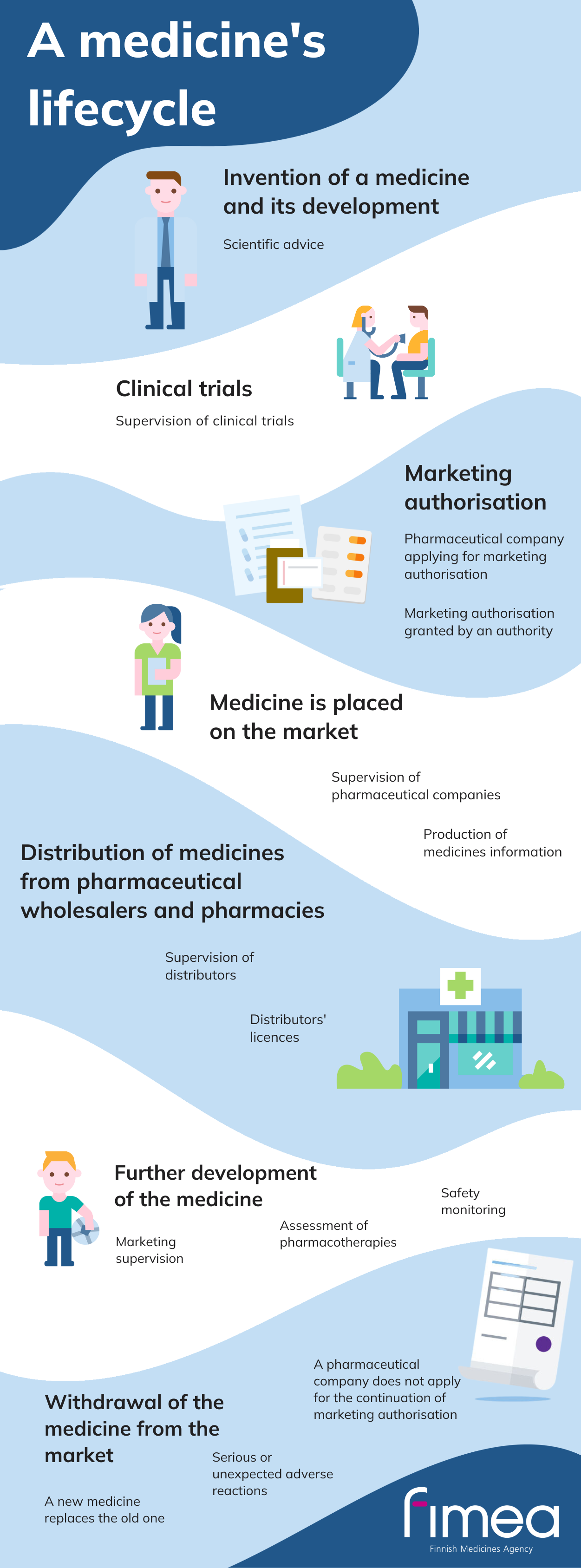
A medicine's lifecycle
- Invention of a medicine and its development: scientific advice
- Clinical trials: supervision of clinical trials
- Marketing authorisation: pharmaceutical company applying for marketing authorisation, marketing authorisation granted by an authority
- Medicine is placed on the market: supervision of pharmaceutical companies, production of medicines information
- Distribution of medicines from pharmaceutical wholesalers and pharmacies: supervision of distributors, distributors' licences
- Further development of the medicine: marketing supervision, assessment of pharmacotherapies, safety monitoring
- Withdrawal of the medicine from the market: a new medicine replaces the old one, serious or unexpected adverse reactions, a pharmaceutical company does not apply for the continuation of marketing authorisation
Fimea web Medicines search: the Medicines search provides information on all medicinal products in Finland that have marketing authorisation.
Sic! 3-4/2017: Fimea ensures the availability of medicines (Julkari, in Finnish)