Information for health care professionals
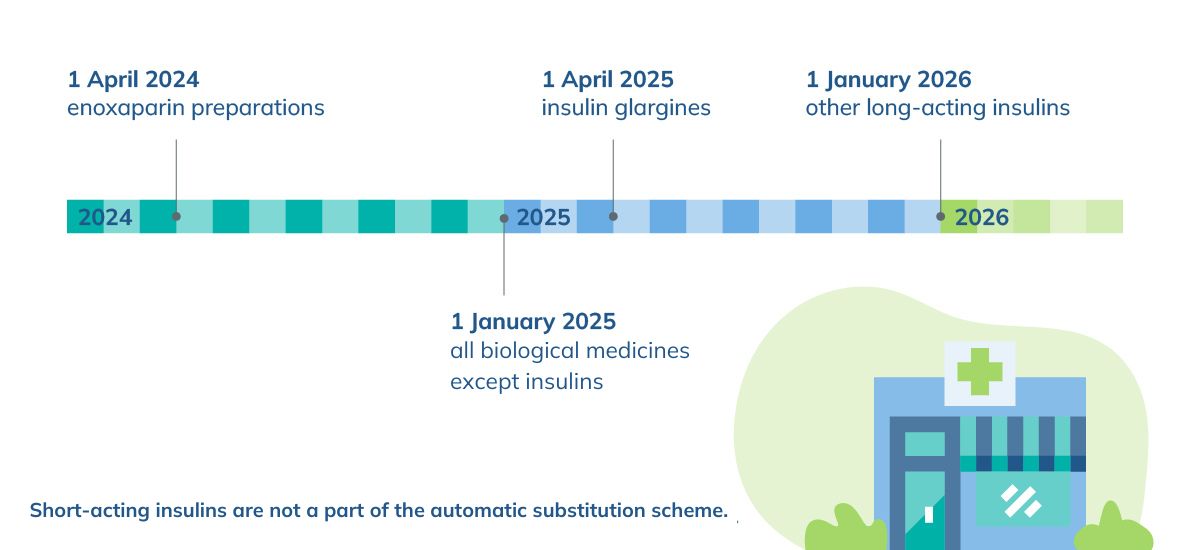
A biological medicine can be substituted at a pharmacy with a more affordable alternative. The automatic substitution is carried out gradually, starting with enoxaparin products and other low molecular weight heparins belonging to the same medicine group. The long-acting insulins was substituted in final phase in January 2026. Short-acting insulins are not a part of the automatic substitution scheme. Biological medicines for patients under the age of 18 will not be substituted at a pharmacy. Automatic substitution increases price competition and curbs the medicine reimbursement costs of the society.
Since 2017, physicians have had a obligation to prescribe the most affordable biological medicine suitable for the patient's care. Prescriptions for biological medicines are only valid for one year at a time.