Promoting access to medicines and medical products

UN Sustainable Development Goal 3:

3.4 By 2030, reduce premature deaths from non-communicable diseases by one third through prevention and treatment, and promote mental health and well-being.
3.8 Achieve universal health care coverage, including financial risk protection, access to high-quality and essential health-care services, and safe, effective, high-quality and affordable essential medicines and vaccines for all.
Challenge
The United Nations (UN) has set mortality from cardiovascular diseases, cancers, diabetes and respiratory diseases as one of the indicators of healthy life and well-being. The World Health Organization (WHO) has also published an action programme for the prevention of chronic non-communicable diseases. For example, cardiovascular diseases, diabetes, asthma and allergies as well as cancer are considered chronic, i.e. long-term, and endemic non-communicable diseases in Finland. However, there are effective medicines for these diseases.
Shortages in medicines is a globally growing phenomenon, and small market areas such as Finland also face challenges in the availability of medicines. The number of shortages reported to Fimea has increased over the past ten years. Some of the problems in accessibility are caused by factors that Finland has very limited influence on. These include the globalisation of pharmaceutical and raw material manufacturing and complex pharmaceutical chains. Shortages may be due to production or logistics problems, shortages of raw materials, unexpected fluctuations in demand or commercial reasons. In most cases, breaks in the availability of medicines are not visible to the patient, as a similar product (within the scope of generic substitution) that can be supplied by the pharmacy is often available.
It is the responsibility of the marketing authorisation holder of the medicine, i.e. the pharmaceutical company, to ensure that the authorised product is continuously available to wholesalers and pharmacies for the needs of patients and other users. This is also a legal condition attached to the granting of a marketing authorisation.
Fimea’s role
The evaluation of medicines and ensuring that they are manufactured and distributed appropriately is Fimea’s basic task, which we supervise and promote in many different ways. Fimea grants marketing authorisations for human and veterinary medicines both as a national authority and in cooperation with other EU pharmaceutical authorities. Fimea also supervises the regulatory compliance of medical devices and the operators in the sector in Finland. In addition, Fimea ensures the adequacy of pharmacy services, the reliability of the supply and distribution of medicines and effective price competition in order to safeguard the consumer's position.
Fimea actively influences the EU’s pharmaceutical strategy and supervision. Effective supervision ensures the safety and quality of medicines and the appropriateness of distribution and deliveries. Appropriately targeted, efficient and effective supervision of pharmaceutical services and medical device operators is the basis for ensuring patient safety and human and animal well-being.
Fimea’s objective
Fimea aims to promote the health and wellbeing of citizens in a sustainable manner and to ensure that safe, efficient, high-quality and affordable essential medicines and vaccinations are available to citizens. Fimea also strives to be the primary and up-to-date source of information on shortages. The objective is for shortages of medicines and medicinal products to have a minimum impact on patient care at the national level.
How the successful attainment of the objective is promoted by Fimea
- Fimea assesses and grants marketing authorisations for medicinal products intended for humans and animals. The number of evaluations carried out by Fimea as the responsible rapporteur or co-rapporteur country in the centralised authorisation procedure coordinated by EMA is significant in relation to the agency's size, which increases Fimea’s international impact. Fimea is a pioneer in particular in the assessment of marketing authorisations for biosimilars: As a responsible reporting country, Finland has assessed the second highest number of marketing authorisation applications for biosimilars from EU countries in 2023.
- In its strategy, Fimea emphasises securing the availability of generic products and increasing their selection. Fimea has continued to invest in accessibility to generic products by acting as the reference Member State responsible for them in a significant part of the EU’s joint application processing.
| Medicinal product Sort the table ascending by the column | 2018 Sort the table ascending by the column | 2019 Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|---|---|---|
| Human medicine | 9 224 | 9 515 | 9 584 | 9 867 | 10 059 | 10 311 |
| Veterinary medicine | 1 030 | 1 080 | 1 131 | 1 163 | 1 192 | 1 215 |
| Total | 10 254 | 10 595 | 10 715 | 11 030 | 11 252 | 11 526 |
- Fimea uses legislation to encourage the development of medicines for children in order to improve the availability of paediatric medicines.
- Fimea ensures the availability of medicinal products needed for sexual and reproductive health (such as contraception, the treatment of infertility and sexually transmitted diseases), which also promotes gender equality and non-discrimination.
- Fimea supervises the regulatory conformance of medical products and the operators in the sector. The supervision of the regulatory compliance of devices pertains to medical devices placed on the market as well as their professional use and maintenance. Fimea grants medical devices sales certificates of free sale, research permits and exemptions.
Notified bodies for medical devices
Before medical devices enter the European market, they must pass the conformity assessment described in EU regulations. Unlike medicines, CE-labelled medical devices do not have a marketing authorisation granted by the authorities but are assessed by notified bodies. Notified bodies are independent parties carrying out conformity assessment. Fimea appoints the notified bodies operating in Finland and supervises their operations.
Notified bodies play a critical role in the market entry process. They are an important part of the system that ensures the availability, quality and safety of medical devices. There are currently two notified bodies operating in Finland that are in accordance with the Medical Device Regulation (MDR EU 2017/745), and in 2023, two notified bodies that are in accordance with the In Vitro Diagnostic Medical Devices Regulation (IVDR EU 2017/746) were also established in Finland. There are 40 notified bodies throughout the EU for MDR-compliant devices and 12 notified bodies for IVDR-compliant devices.
- Fimea supervises the safety of products of human origin, such as blood and blood products, tissues, organ transplants and advanced therapy medicinal products (ATMPs) and operators in the field. Fimea grants licences for blood and tissue establishments and is responsible for regular inspections of the establishments.
Case example: Reform of the criteria for the approval of blood donors
Fimea's revised regulation on the activities performed by blood service entered into force in October 2023.
The new regulation amended the criteria for those eligible to donate blood so that the temporary ban on blood donation after sex between men was lifted. Sex with new, different or multiple sexual partners always causes a four-month temporary ban on blood donations.
In addition, a temporary ban on blood donation was still imposed in the event certain infections or exposure to these, exposure to a disease communicable by blood, and certain vaccinations.
The elimination of the temporary ban on blood donations due to sex between men is important for the equality of citizens. People living in a long-term, established relationship can now donate blood regardless of their sexual orientation.
- Fimea grants licences for pharmacies, national pharmaceutical factories and pharmaceutical wholesalers and inspects operators regularly. In addition to domestic pharmaceutical factories, Fimea also inspects pharmaceutical factories in third countries that import medicines into the EU/EEA. To ensure that each sector is adequately supervised, Fimea monitors the number of inspections carried out linked to different actors on an annual basis.
| Year Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|
| Pharmaceutical manufacturers GMP | 30 | 31 | 34 |
| Blood services | 4 | 6 | 5 |
| Hospital pharmacies and pharmaceutical centres | 18 | 18 | 14 |
| Pharmacies and subsidiary pharmacies | 28 | 36 | 55 |
| Pharmaceutical wholesalers | 25 | 27 | 26 |
| Tissue establishments | 25 | 25 | 22 |
| Organ donation and transplantation services | 0 | 6 | 6 |
| Inspections concerning manufacturers of medical devices | 9 | 9 | 19 |
| Inspection of authorised representatives, importers and distributors of medical devices | 2 | 3 | 4 |
| Inspection of the site of f clinical trials of medical devices | 0 | 2 | 1 |
| Annual monitoring of the notified body | 3 | 3 | 3 |
| Inspection of a sterilisation service provider | 0 | 0 | 0 |
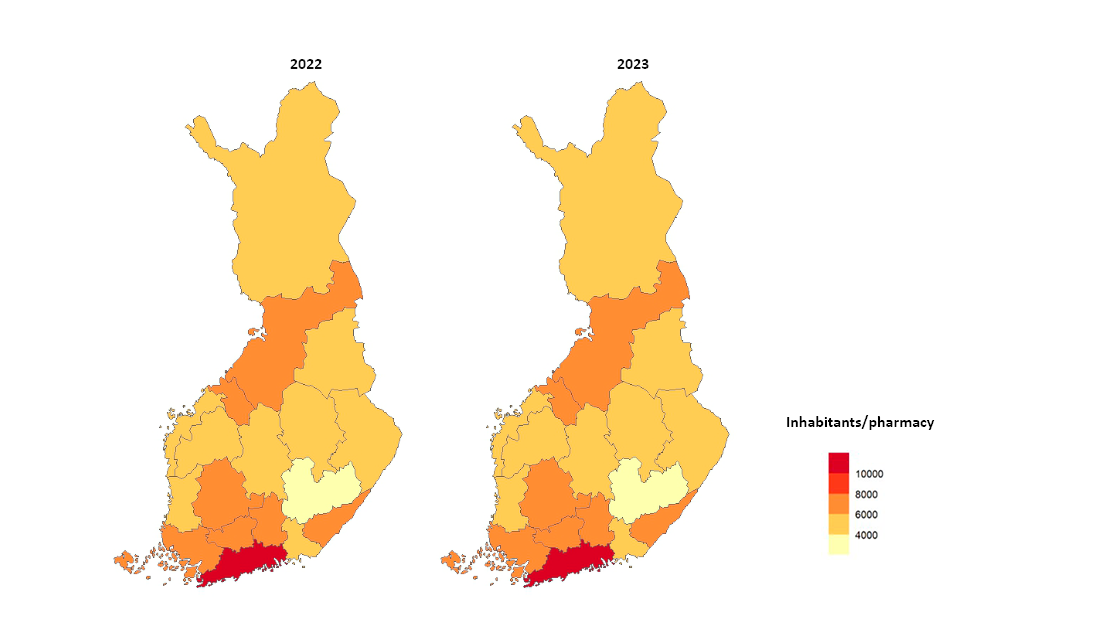
- In Finland, the number of operators in pharmacy services is restricted. Pharmacies will develop new service forms, such as online pharmacy services and home delivery services, which promote the availability of medicines. Fimea ensures that the Finnish pharmacy network is sufficiently comprehensive, meaning the pharmacy network evolves according to customer needs and medicines are adequately available also in remote areas. Fimea annually assesses the coverage of the distribution network and monitors the number of pharmacy outlets per municipality resident. A total of 640 pharmacies and 189 subsidiary pharmacies operated in Finland on 31 December 2023. New pharmacists took over 107 pharmacies and a first pharmacist was selected for seven pharmacies previously established by Fimea.
- Fimea is manages the comprehensive range of medicines required by healthcare and monitors the number of marketing authorisations granted and the pricing policy of medicines. For example, Fimea maintains a list of interchangeable medicinal products. Interchangeable medicines contain an equal amount of the same active medicinal substance, which means the medicine can be exchanged at the pharmacy for the cheapest available interchangeable medicine. Products with lower prices, such as generic medicines and biosimilars, enable the availability of economically sustainable medicines and health benefits for the widest possible population. From the beginning of April 2023, inhaled medicinal products used for the treatment of asthma and COPD became interchangeable. The exchange of medicines was enhanced to increase price competition between products thus improving the cost-effectiveness of pharmaceutical services.
| Amount Sort the table ascending by the column | 2017 Sort the table ascending by the column | 2018 Sort the table ascending by the column | 2019 Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column |
|---|---|---|---|---|---|---|
| Total, EUR million | 70,1 | 77,8 | 75,5 | 75,1 | 78,2 | 102,3 |
| Savings/substitution, EUR | 23,7 | 22,9 | 19,0 | 18,2 | 16,7 | 21,2 |
Sources: Kela's material Dispensed medicines reimbursable under the National Health Insurance scheme. Price and reference price data from the Pharmaceutical Database. Compensation includes additional compensation. The statistics also cover purchases made prior to the fulfilment of the initial deductible.
Savings brought about by changes in medicines in 2017–2022 in an accessible format (pdf).
- The pharmaceutical company is responsible for the information on the availability of the medicine and for its introduction to the market. Where necessary, Fimea supervises and investigates shortages of medicines with pharmaceutical companies. Fimea maintains a list of current shortages on its website, which is based on information provided by pharmaceutical companies. In 2023, Fimea introduced a register and e-services for the processing of shortage notifications submitted by pharmaceutical companies. The reporting of the situational picture on the availability of medicines and preparedness will be developed in 2024. Information on different sources of information on availability, also taking into account the information on shortage notifications, will be compiled into reports for the needs of national and EU authorities.
Fimea actively works in the European Medicines Agency's (EMA) medicines availability management networks and working groups. In 2023, Fimea participated in the preparation of the EU list of critical medicines, which aims to prevent supply problems particularly harmful to patient care. A medicine is considered critical if it is used for a serious condition and no substitute can be easily found.
In 2023, Fimea led one subproject of the EU cooperative project. The aim of the subproject was to prevent disruptions in availability and prevent their impact on patients. The aim is to harmonise availability management of medicines among medicines authorities to minimise the impact of availability disruptions on patient treatment.
In 2023, Fimea also enhanced the internal sharing of information related to shortages and the availability of medicines. - Fimea participates in cooperation in the management of the availability of medical devices. In June 2023, the European Medicines Agency (EMA) established a Medical Device Shortages Single Point of Contact (SPOC) working group, which Fimea also joined.
The SPOC working group was set up to make recommendations to the Executive Steering Group on Shortages of Medical Devices (MDSSG) on issues directly or indirectly related to shortages or availability problems of medical devices during public health emergencies. In 2023, a joint EU user interface was developed for the monitoring and reporting of equipment shortages. - Fimea supervises the mandatory reserve of medical supplies, the purpose of which is to ensure the availability of medicines in disruptions, which have hindered or prevented the normal availability of medicines to our country, due to e.g. interruptions in the supply of medicines or a serious crisis. Where necessary, Fimea can grant or propose that the Ministry of Social Affairs and Health grants exemptions allowing pharmaceutical companies to maintain lower stock of mandatory reserve medical supplies to secure the uninterrupted distribution of medicinal products or allow them to have no mandatory reserve medical supplies.
The realisation of mandatory reserve medical supplies is supervised in inspections of operators subject to mandatory reserve medical supply storage obligations. Pharmacies are obliged to store a quantity of medicinal products corresponding to the consumption of their normal customer base over a two week period. Fimea supervises this in pharmacy inspections. - Fimea participates in national preparedness activities. Fimea participated in the activities of the Advisory Board for Health and Welfare in Emergency Conditions (Advisory Board for Health and Welfare in Emergency Conditions ) in matter related to both medicines and medical devices. The advisory board was appointed by the Ministry of Social Affairs and Health to promote preparedness for emergency conditions in social and health care and environmental health care. The Advisory Board prepares proposals on health care and social welfare arrangements for normal conditions that will also support activities in emergency conditions.
- To ensure the uninterrupted continuation of pharmacotherapy, Fimea grants special permits or exemptions in situations where a critical medicinal product with marketing authorisation is not available in Finland. The special permit procedure ensures the medical treatment of an individual patient in exceptional cases by granting a permit for a medicinal product that does not have a marketing authorisation in Finland for special medical reasons.
| Transaction Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|---|
| Shortage notifications | 2 093 | 1 710 | 2 335 | 2 849 |
| Decisions on mandatory reserve medical supply storage | 391 | 313 | 478 | 494 |
| Decisions on exemption to maintain lower reserve medical supplies | 312 | 270 | 441 | 450 |
| Applications for an exemption received (human medicines)* | 198 | 125 | 175 | 200 |
| Exemption decisions (human medicines)* | 189 | 107 | 152 | 186 |
| Applications for an exemption received (veterinary medicines)* | 33 | 49 | 60 | 46 |
| Exemption decisions (veterinary medicines)* | 31 | 46 | 53 | 39 |
| Patient-specific special permit decisions | 7 327 | 6 809 | 7 402 | 8 917 |
| Institution-specific special permit decisions | 8 465 | 7 730 | 8 019 | 7 926 |
| Fixed-term special permit decisions | − | − | 110 | 81 |
| Exemptions for medical devices (MD Regulation and IVD Regulation) | − | 1 | 14 | 10 |
Fimea monitors various variables that affect the availability of medicines, such as shortage notifications, the number of exemptions to lower the stock of mandatory reserve medical supplies, and the number of exemptions and special permits.
* For exemptions, the method of calculation has changed, which is why the figures are not directly comparable with previous years’ figures.
Number of payments related to the management of the availability of medicines and medical devices in 2020–2023 in an accessible format (pdf).