Accidents involving poisoning and exposure to harmful substances:
- Accidental overdose or accidental administration or taking of an incorrect medicine.
- Accidents involving the use of medicinal substances, medicines or biological substances in connection with surgical or other medical treatment.
- Poisoning where it has not been determined whether it was accidental or with the intention of harm
Source: WHO (Global and Europe, Statistics Finland (Finland).
Mortality due to unintended poisoning (per 100,000 persons) in an accessible format (pdf).
Safety of medicines and medicinal products and production of reliable medicines information

UN Sustainable Development Goal 3:

3.5 Enhance the prevention and treatment of substance abuse, including drugs and alcohol.
3.8 Achieve universal health care coverage, including financial risk protection, access to high-quality and essential health-care services, and safe, effective, high-quality and affordable essential medicines and vaccines for all.
3.9 By 2030, significantly reduce deaths and diseases caused by hazardous chemicals and air, water and soil contamination and pollution
Challenge
The safety of medicines or medicinal products is vital, as is reliable information on these. However, the spread of misinformation on medicines through different communication channels is a growing concern. Abuse of medicines, such as unintended overdose of medicines or taking the wrong medicine, can have harmful or even fatal consequences. The United Nations (UN) has selected mortality associated with unintended poisoning as one of the indicators of healthy life and well-being.
Despite quality assurance, errors may occur in the manufacture of medicines, resulting in the sale of medicines which do not meet quality requirements or might be hazardous to users. Furthermore, counterfeit medicines are a global problem, especially in countries with poor pharmacovigilance. Counterfeit products may contain incorrect, ineffective or even life-threatening substances. In Finland, Fimea supervises medicines and medical products and their safety.
Fimea’s role
Fimea plays an important role in producing reliable, clear and up-to-date information related to medicines, medical products and pharmacotherapies. Fimea produces unbiased information for citizens, professionals in the healthcare and social welfare sector and others in need of medicines information. Fimea’s tasks also include the long-term planning and coordination of medicines information and the sharing of reliable medicines information.
The efficacy, safety, risk-benefit balance and quality of medicines are assessed as part of the marketing authorisation process. Fimea also supervises the regulatory compliance of medical devices and the operators in the sector in Finland. The safety of medicines is monitored and assessed throughout their life cycle.
Fimea protects public health by monitoring the import, export, manufacture and distribution of narcotics, thus aiming to prevent their misuse. Fimea also assesses the properties of new psychoactive substances used in a similar manner as narcotics. Fimea's role in the supervision of narcotics is important, but only one factor among other measures to reduce the negative impacts to health caused by the abuse of narcotics.
Objective
Fimea’s objective is to be the primary source of pharmaceutical information in Finland. Achieving objectives requires anticipating, systematic and target group-oriented communication as well as continuous interaction and cooperation with stakeholders. The aim is to promote the well-being of citizens through safe medicines and medicinal products and to reduce the health risks posed to citizens by substances used as narcotics.
How the successful attainment of the objective is promoted
Fimea provides reliable information on medicines and medicinal products on its website and in various social media.
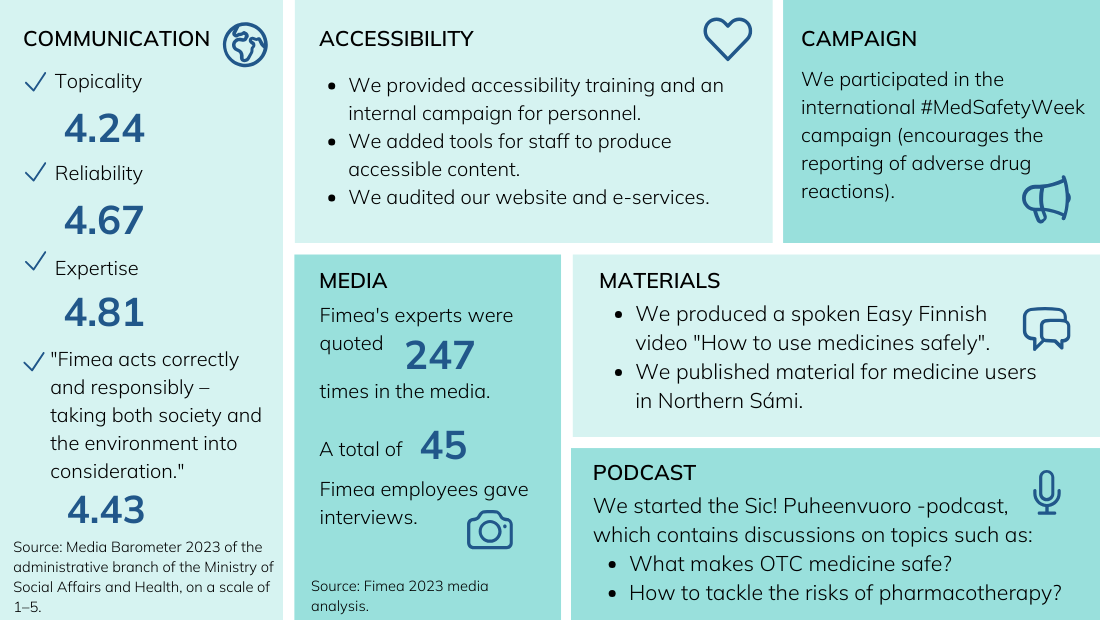
Fimea has promoted the achievement of its objectives in the following manner:
- Offering information products based on evidence and expert opinion to support the work of health care professionals. With the help of the information products offered by Fimea, the management of wellbeing services counties as well as health care professionals have up-to-date evidence-based information.
- The purpose of Meds75+ is to support the clinical decision-making on the pharmacotherapy of patients over 75 years of age and to improve medication safety in primary health care.
In 2023 Fimea carried out the following with regard to Meds75+- invested in international language versions by updating the Swedish and English texts to better match medical terminology.
- was present at two international congresses and two Finnish congresses.
- conducted user interviews on the quality and structure of information content as part of the information management and maintenance development project. As part of the development project, a new digital workspace was also developed for the expert group responsible for maintenance.
- In April 2023, Fimea published the National Risk Medicines Classification. The medicines classified as high risk are those the use of which requires special attention because their incorrect use may have serious consequences for the patient. The classification, which describes the serious consequences of medicines and treatment risk points, helps to identify key high-risk medicines and ensure their safe use at different stages of the pharmacotherapy process.
- Fimea introduced a renewed medicines wholesale register in 2023. The reporting tool can be used to produce automated, visualised and informative reports on the register. Fimea uses the wholesale register to monitor and compile statistics on the sales and consumption of medicines and uses this information to compile a knowledge base on medicines consumption in Finland. The new register and the Power BI reporting developed for it facilitate knowledge-based management in supervision, guidance and research, as well as the planning and analysis tasks of the authorities.
- In early 2023, the contents of the Medicines Barometer 2023 population survey (www.julkari.fi, in Finnish) were planned in cooperation with stakeholders. Current themes related to medicines were selected as the subject of the survey. The survey examined the long-term illnesses of Finns aged 18–79 and the implementation and monitoring of pharmacotherapy for these, participation in decision-making on pharmacotherapy, experiences of medicines advice, the most used sources of medicine information, health literacy, views on the locations where medicines are purchased, views on vaccines, attitudes on environmental issues related to medicines and commitment to pharmacotherapy.
- In 2023, Fimea produced medication monitoring data on the population aged 75 or over. These indicators can be used both nationally and in wellbeing services counties to assess the use and costs of medicines. Fimea also monitors the savings potential of medicines dispensed that are reimbursed nationally from the health insurance of pharmacies. We could save tens of millions of euros each year in the cost of medicines, if cheaper interchangeable products were used.
- The purpose of Meds75+ is to support the clinical decision-making on the pharmacotherapy of patients over 75 years of age and to improve medication safety in primary health care.
More than one third of those aged 75 or over use medicines the use of which should be avoided by older people. The total number of people using medicines that should be avoided in accordance with recommendations has increased annually due to the increase in the number of older people. During the review period, the share of older people using multiple medications, i.e. at least 10 different medicines, has been one fifth of all those aged 75 or over, and the share of those using multiple medications has increased annually. At the same time, the total costs of medicines dispensed by prescription for patients aged 75 or over have increased throughout Finland.
Source: www.fimea.fi
Indicators describing safe pharmacotherapy for older people in 2020-2023 in an accessible format (pdf).
- By coordinating the activities of the Medicines Information Network, which involves approximately 65 pharmaceutical sector operators from Finland. The operation of the national Medicines Information Network is based on "The Medicine User at the Centre of Medicines Information – National Medicines Information Strategy 2021‒2026". The working groups in the Medicines Information Network have launched a number of measures of varying scopes, the aim of which has been e.g. to produce and communicate reliable information on medicines to both medicine users, and healthcare and social welfare professionals. In addition to the new materials, several events have been organised, such as the annual Pharmacotherapy Day. The actors in the Medicines Information Network also participate actively in working groups for national development, most recently in the preparation of the medicines data repository.
- By participating in the translation of the medical dictionary MedDRA. MedDRA (Medical Dictionary for Regulatory Activities) is a glossary developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) e.g. for the classification of adverse reactions to medicines. In 2023, a team of Fimea experts assisted in producing the Finnish version of the glossary by guiding the principles of translation work and reviewing more than 20,000 terms from the glossary. The high-quality translation of the glossary into Finnish will help in making summary of product characteristics and information on packages concerning adverse reactions easier to understand for the parties prescribing medicines, their users and suppliers.
- By guiding and supervising pharmaceutical advice provided by pharmacies.
- The substitution of inhaled medicines used to treat asthma and COPD for a cheaper product began on 1 April 2023 in pharmacies. In this context, Fimea instructed pharmacies, for example, with a guidance letter and by organising a training webinar on the topic. Preparations for the supervision of the pharmacy exchange of biological medicines were also started. From April 2024 onwards, it has been possible for a pharmacy to substitute a biological medicine prescribed to the patient with a cheaper interchangeable medicinal product.
- In addition, supervision was particularly focused on the implementation of advice on OTC and prescription medicines by pharmacies. Supervision focused particularly on personnel competence and the availability of facilities, tools and materials for suitable for the provision of advisory services. In addition, it was inspected which principles on pharmaceutical advice had been agreed upon and how pharmaceutical personnel had been instructed on them.
- Monitoring the safety of medicines and medicinal products. In 2023, e.g.:
- Fimea's adverse reaction database and electronic reporting channel for adverse reactions were reformed. In the future, this will make it possible to work on adverse reaction reports regardless of location and in a paper-free environment. In addition, the new electronic reporting channel is more streamlined and provides better instructions for reporting (healthcare professionals and medicine users).
- Fimea's pharmaceutical safety assessment division participated in the assessment of centralised marketing authorisations, annual reports on different types of marketing authorisation obligations and periodic safety reviews of medicinal products.
- Fimea was assigned six new rapporteurships by the Pharmacovigilance Risk Assessment Committee (PRAC), which is responsible for the risk management and post-market surveillance of medicinal products.
- In 2023, Fimea assessed seven studies on the combined use of medicines and devices. Six of these were performance studies and one was a study in which the device administered the medicine the study concerned. In a webinar held in May 2023, stakeholders were provided training on the special features of combination trials. Joint assessment-related matters are discussed within Fimea on a monthly basis.
- Fimea participated in an EU Joint Action on Reinforced Market Surveillance of Medical Devices and In Vitro Medical Devices (JAMS 2.0) the purpose of which is to develop and harmonise market surveillance in the European Union's internal market so that only safe medical devices that meet the requirements are available on the market.
- Being on-call in matters concerning product defects in medicines 24/7 every day of the year. Pharmaceutical sector operators are responsible for the measures required for product defects, but Fimea supervises that the measures are adequate and appropriate. Fimea takes part on the European regulatory network to prevent the distribution of counterfeit medicines and medicinal products.
- By supervising serious quality and safety deviations that apply to blood products, tissue and organ transplants.
Case example: Fimea warns of counterfeit medicines
Fimea warned consumers twice in 2023 about counterfeits of so-called new-generation diabetes medicines. Counterfeit versions of products appeared on the black market. These products were experiencing global availability problems in the legitimate market due to increased demand. There is no guarantee of the content and quality of medicines obtained from illegal websites or other unreliable marketplaces. For example, in the spring and summer of 2023, there were cases in Switzerland in which a user of an illegally obtained medicine had been hospitalised as a result of the use of a product that proved to be insulin but the user believed to be another product.
The safety features of prescription packages were crucial in the detection of counterfeit Ozempic products found in Europe in autumn 2023. Counterfeit packaging was detected in the control of safety features both in the European Union and in Great Britain. The pre-filled pens in packaging from central European pharmaceutical wholesalers differed in appearance from the original. It was later confirmed that the pre-filled pens were the insulin pens of different manufacturers. The packages did not reach the patients. Counterfeit packaging has not existed in the legal distribution chain of medicines in Finland.
- By supervising pharmaceutical sector operators by examining the quality of medicines used in Finland. For example, Fimea's inspectors will take pharmaceutical samples for analysis during pharmaceutical industry and pharmacy inspections. The aim of Fimea's laboratory quality control is to ensure that medicines intended for humans and animals are of high quality and safe. Fimea's laboratory moved to new premises in 2023, which will make it possible to carry out laboratory tests that meet the continuously evolving monitoring needs of the pharmaceutical sector better than before.
- By carrying out control campaigns in the laboratory in which the essential quality characteristics of generic products containing a particular active medicinal substance are tested simultaneously. Pharmaceutical products that are widely used in Finland are selected for control campaigns. Such control campaigns provide a good situational picture of the quality of the products in the group in question in Finland. In the 2023 control campaign, all pills of different strengths containing olanzapine (a psychotic drug) and film-coated pills sold on the Finnish market were tested. There were no comments on the quality of the products tested.
- Monitoring the medical use of narcotics In 2023, for the first time ever, medicinal products already on the market were added to the new national control category when the control category for pregabalin (pharmaceutical used to treat epilepsy and peripheral neuropathic pain) was changed. The change was made due to the long-standing abuse of pregabalin. Its purpose is to prevent the use of pregabalin as an intoxicant and the resulting adverse effects. The change in the control class of products on the market and in pharmaceutical use required a great deal of preparation, and pharmacies, for example, were instructed to monitor the dispensing of medicines suitable for misuse.