Advice on appropriate pharmaceutical marketing
All Topics
Ensuring the quality of pharmaceutical marketing
The line between medicines information and pharmaceutical marketing
Take these things into account in pharmaceutical marketing
The inverted black triangle and standardised explanatory sentence in pharmaceutical marketing
Over-the-counter medicine marketing checklist
Prescription medicine marketing checklist
Regulations on pharmaceutical marketing
Ensuring the quality of pharmaceutical marketing
Under section 94 of the Medicines Act, the holder of a marketing authorisation for a medicinal product or the registration of a traditional herbal medicinal product must have a scientific service unit responsible for the information provided in the marketing of the medicinal product.
Quality assurance process
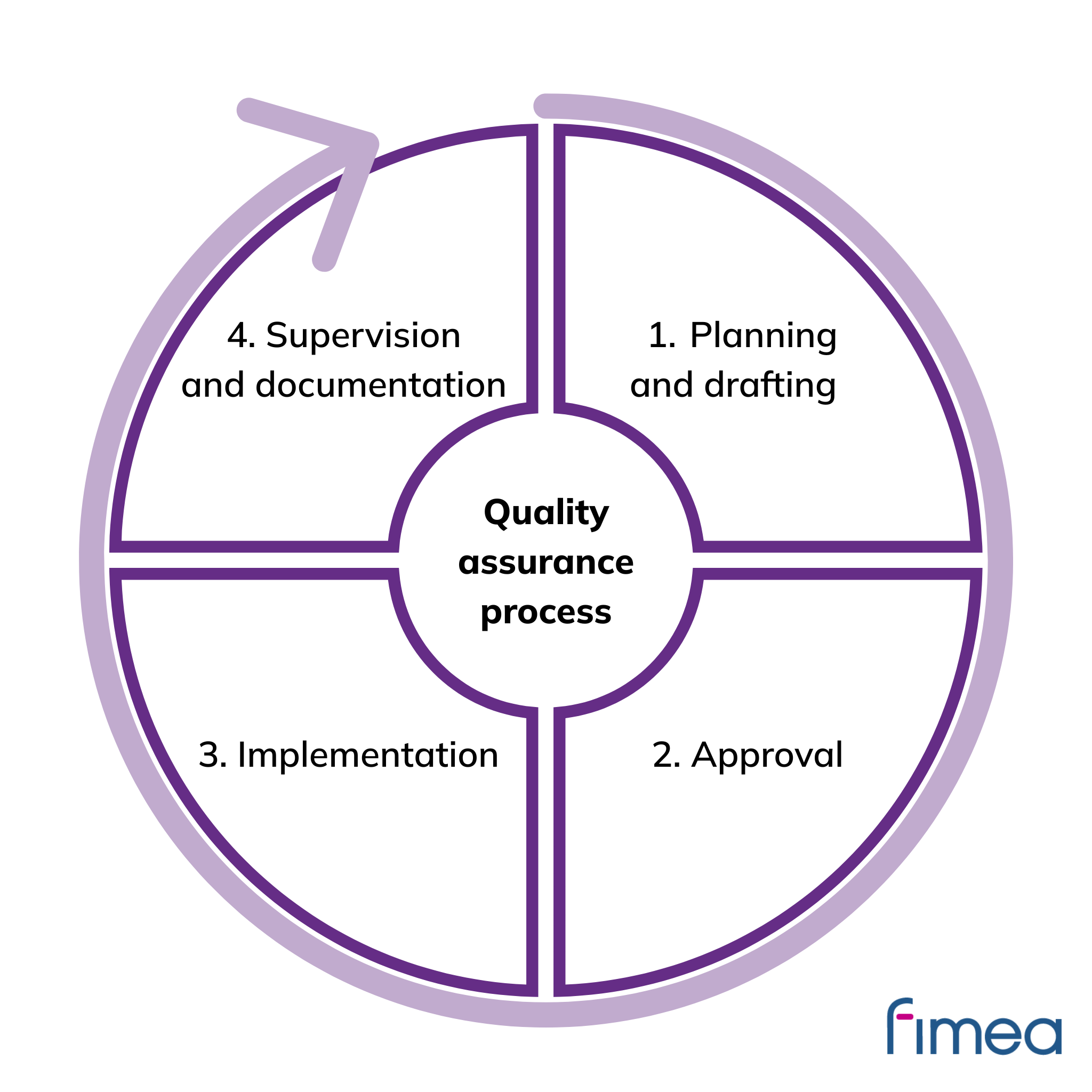
1. Planning and drafting
A line is drawn between distribution of information and advertising in accordance with legislation and instructions.
2. Approval
The approver checks the legality of the advertisement or communication. Instructions define the responsibilities and obligations concerning the commenter and the approver.
3. Implementation
Reasonable hospitality and visibility of the information content only to the legal target group are ensured.
4. Supervision and documentation
The approval and implementation process is recorded and archived systematically, including up-to-date information on dates and target groups, for example.
The line between medicines information and pharmaceutical marketing
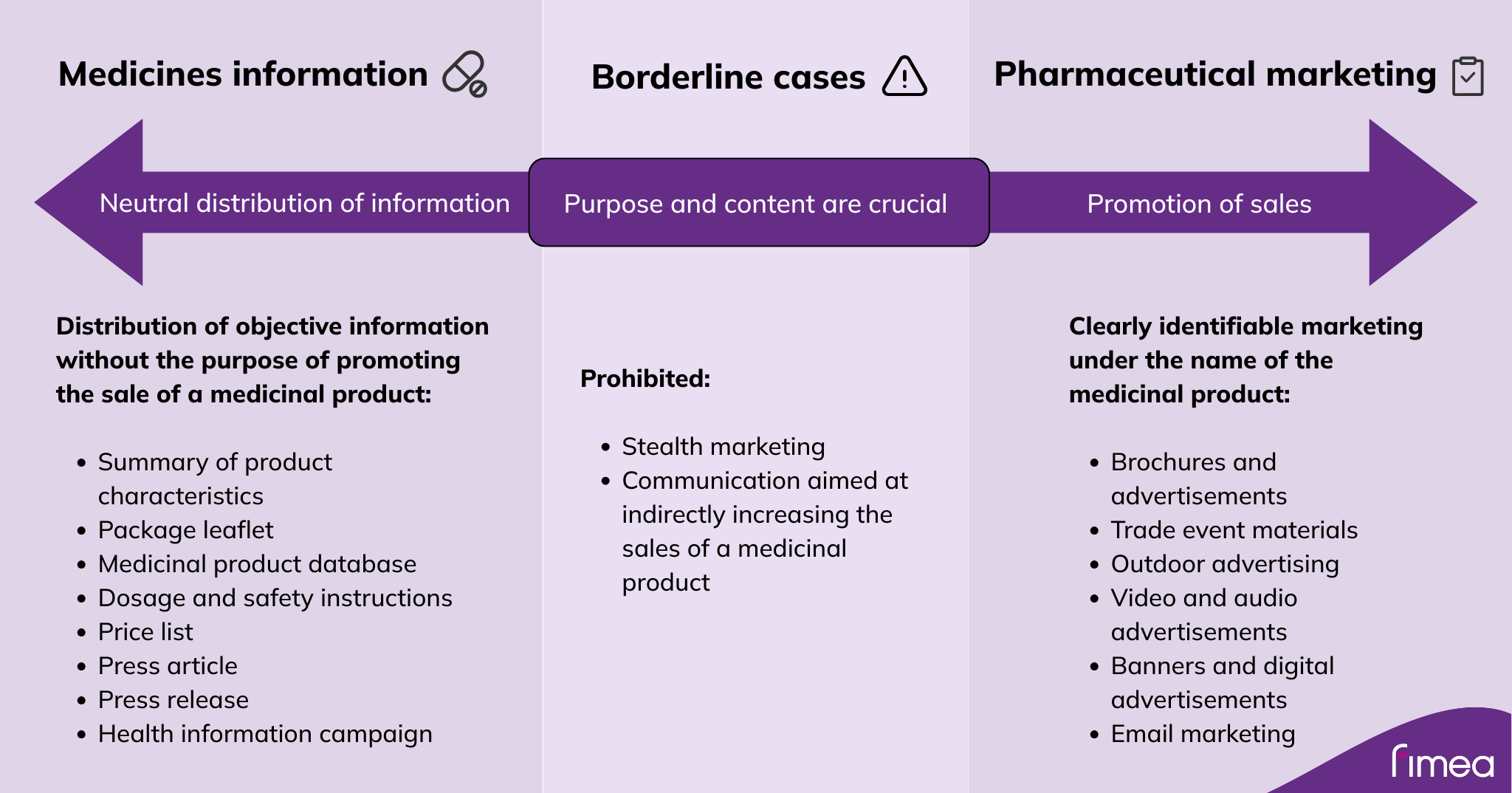
Fimea recommends that the holder of a marketing authorisation or registration inform Fimea if it becomes aware that an external party is presenting material that is considered marketing for their medicinal product. This applies especially to situations that can be seen as marketing a prescription medicine to the general population. Marketing a prescription medicine to the general population is not allowed. It is advisable to mention in the notification whether the company has any interests, agreements or cooperation with the party in question. A proactive notification helps avoid situations in which Fimea would have to request clarification from the company.
Take these things into account in pharmaceutical marketing
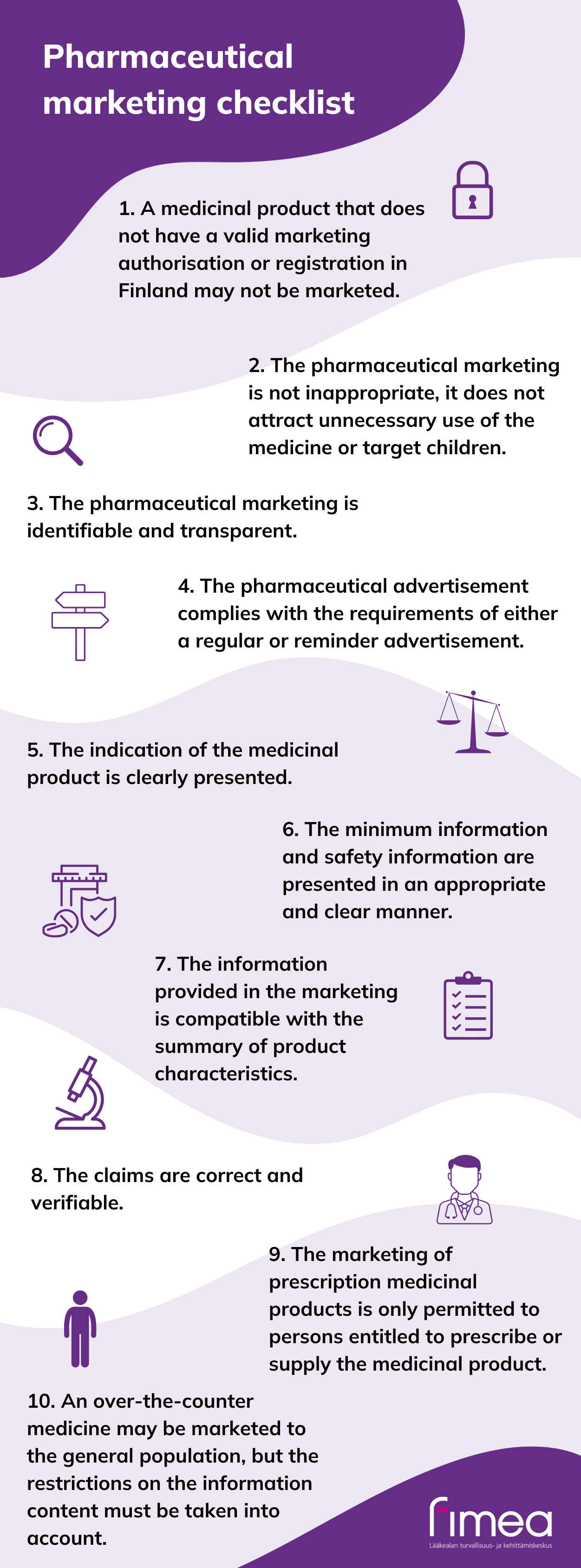
See below for more detailed instructions on how to ensure that over-the-counter and prescription medicinal products are marketed in accordance with norms as well as references to the relevant regulations.
