Fimea monitors various variables that affect the availability of medicines, such as shortage notifications, the number of exemptions to lower the stock of mandatory reserve medical supplies, and the number of exemptions and special permits.
* For exemptions, the method of calculation has changed, which is why the figures after 2022 are not directly comparable with previous years’ figures.
Promoting access to medicines and medical products

UN Sustainable Development Goal 3:

3.4 By 2030, reduce premature deaths from non-communicable diseases by one third through prevention and treatment, and promote mental health and well-being.
3.8 Achieve universal health care coverage, including financial risk protection, access to high-quality and essential health-care services, and safe, effective, high-quality and affordable essential medicines and vaccines for all.
Challenge
The United Nations (UN) and the World Health Organisation (WHO) have defined that mortality from cardiovascular diseases, cancers, diabetes and respiratory diseases is one of the indicators of healthy life and well-being. In Finland, cardiovascular diseases, diabetes, asthma and allergies as well as cancer are considered chronic endemic diseases. Appropriate medicinal treatment helps to manage and slow down the symptoms of chronic illness and improves quality of life for the affected person.
The current global situation poses challenges in accessing medicines and medical products. The after-effects of the pandemic, fluctuations in demand, new production requirements and temporary production interruptions are examples of factors affecting availability. In most cases, breaks in the availability of medicines are not evident to patients, as a similar product within the scope of generic substitution that can be supplied by the pharmacy is often available.
It is the responsibility of the marketing authorisation holder of a medicine, meaning the pharmaceutical company, to ensure that the authorised product is continuously available to wholesalers and pharmacies for the needs of patients and other users. This is also a statutory condition attached to the granting of a marketing authorisation for a medicine.
Fimea’s role
Fimea’s task is to ensure the appropriate manufacture and distribution of medicines. Fimea grants marketing authorisations for human and veterinary medicines and supervises the compliance of medical devices and operators in the field in Finland. In addition, Fimea ensures the adequacy of pharmacy services, the reliability of the supply and distribution of medicines and effective price competition in order to safeguard the consumer’s position.
Fimea’s objective
One of Fimea’s objectives is to promote the health and well-being of the population in a sustainable manner. Another objective is to make safe, high-quality and affordable medicines and medical products available to citizens when they need them. Fimea strives to be the primary and up-to-date source of information on shortages. The objective is for shortages of medicines and medical products to have a minimal impact on patient care at the national level.
How is Fimea promoting the successful attainment of its objectives?
| Medicinal product Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column | 2024 Sort the table ascending by the column |
|---|---|---|---|---|---|
| Human medicine | 9 584 | 9 867 | 10 059 | 10 311 | 10 757 |
| Veterinary medicine | 1 131 | 1 163 | 1 192 | 1 215 | 1 278 |
| Total | 10 715 | 11 030 | 11 252 | 11 526 | 12 035 |
Pharmaceutical companies are responsible for the availability and marketing of medicines. Fimea supervises potential shortages and investigates them in cooperation with pharmaceutical companies. Fimea maintains an up-to-date list of shortages in the supply of medicines on its website and, if necessary, shares information with separate online bulletins. Availability information is regularly shared with pharmaceutical industry operators, the Ministry of Social Affairs and Health and representatives of the EU and Nordic pharmaceutical authorities.
| Transaction Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column | 2024 Sort the table ascending by the column |
|---|---|---|---|---|---|
| Shortage notifications | 2 093 | 1 710 | 2 335 | 2 849 | 2 676 |
| Decisions on mandatory reserve medical supply storage | 391 | 313 | 478 | 494 | 486 |
| Decisions on exemption to maintain lower reserve medical supplies | 312 | 270 | 441 | 450 | 448 |
| Applications for an exemption received (human medicines)* | 198 | 125 | 175 | 200 | 274 |
| Exemption decisions (human medicines)* | 189 | 107 | 152 | 186 | 254 |
| Applications for an exemption received (veterinary medicines)* | 33 | 49 | 60 | 46 | 20 |
| Exemption decisions (veterinary medicines)* | 31 | 46 | 53 | 39 | 19 |
| Patient-specific special permit decisions | 7 327 | 6 809 | 7 402 | 8 917 | 8 805 |
| Institution-specific special permit decisions | 8 465 | 7 730 | 8 019 | 7 926 | 7 598 |
| Fixed-term special permit decisions | − | − | 110 | 81 | 74 |
| Exemptions for medical devices (MD Regulation and IVD Regulation) | − | 1 | 14 | 10 | 6 |
- Fimea manages the comprehensive range of medicines required by health care and monitors the number of marketing authorisations granted and the pricing policy of medicines. In March 2024, Fimea published the results of the Medicines Barometer 2023 population survey (Julkari, in Finnish) conducted in the previous autumn. One purpose of the Medicines Barometer is to monitor how much of the population has financial difficulties in purchasing medicines. Over the past eight years, this number has grown from 16% to 24%.
- Fimea draws up and maintains a list of interchangeable medicines (in Finnish). The list allows a medicine to be exchanged at a pharmacy for a cheaper interchangeable medicine. The aim of this generic substitution is to increase price competition and reduce medicine reimbursement costs. Using more affordable medicines not only benefits society economically but also the population from a health perspective, as it helps more people afford the medicines they need.
| Amount Sort the table ascending by the column | 2019 Sort the table ascending by the column | 2020 Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column |
|---|---|---|---|---|---|
| Total, EUR million | 75,5 | 75,1 | 78,2 | 102,3 | 114,8 |
| Savings/substitution, EUR | 19,0 | 18,2 | 16,7 | 21,2 | 23,2 |
Sources: Kela's material Dispensed medicines reimbursable under the National Health Insurance scheme. Price and reference price data from the Pharmaceutical Database. Compensation includes additional compensation. The statistics also cover purchases made prior to the fulfilment of the initial deductible.
Case study: launching the generic substitution of biological medicines in 2024
The generic substitution of biological medicines became possible in 2024. The generic substitution is being rolled out at pharmacies in stages, starting in April 2024 with a medicines used to treat and prevent blood clots. Fimea defines interchangeable biological medicines four times a year.
In 2024, Fimea communicated extensively about the new pharmacy substitution for biological medicines. Fimea sent a guidance letter to pharmacies and organised a webinar. Communication campaigns about generic substitution were also organised for prescribers and patients. Fimea developed the available knowledge base and piloted the production of videos for the administration devices of biological medicines. In addition, Fimea started supporting pharmacies’ medication counselling with information on the most essential differences between interchangeable biological medicines, such as the operation of administration devices, in the database for pharmacies’ product-specific guidance (in Finnish).
- Fimea has been involved in developing monitoring programmes commissioned by the EMA for the quality assurance of biosimilar products, and one testing programme for biosimilar products was prepared in 2024 under the leadership of the Fimea Laboratory.
- The development of reporting on the situational picture of the availability of medicines and preparedness was started in autumn 2024. Information from different sources, such as shortage notifications, has been compiled into reports for the needs of national and EU authorities.
- Fimea participated actively in the CHESSMEN cooperation project with EU medicines agencies. The aim of the project is to harmonise best practices for preventing medicine shortages and reducing their impacts. Fimea led the project and continued to participate in the assessment of critical medicines in the EU.
- Availability reporting was promoted in the supervision of medical devices. With the new regulation, medical device manufacturers must report to the supervisory authority if they anticipate an interruption or termination of the delivery of a device, which could cause serious harm to patients or public health. Fimea participated in a working group (in Finnish) that prepared a reporting process, form and instructions to enable meeting the reporting obligation in line with the regulation in January 2025. Availability reporting collects proactive information from medical device manufacturers, which is used to ensure that compliant medical devices are available to patients and health care professionals.
- Fimea launched a five-year pilot allowing the approval of Nordic packages that are in English for certain small-scale but vital hospital products. By simplifying production in this way, the availability of certain critical products in the Nordic countries can be improved. Fimea grants a special permit for compassionate use for a medicine without a marketing authorisation in Finland if a product with a marketing authorisation is not available for the treatment of a patient in Finland. In 2024, Fimea developed the electronic ERKKA system for applying for and processing special permits (in Finnish). This system was introduced in February 2025 alongside an update to Fimea’s FimeaWeb, which enabled searching for products that require a special permit.
| Year Sort the table ascending by the column | 2021 Sort the table ascending by the column | 2022 Sort the table ascending by the column | 2023 Sort the table ascending by the column | 2024 Sort the table ascending by the column |
|---|---|---|---|---|
| Pharmaceutical manufacturers GMP | 30 | 31 | 34 | 40 |
| Blood services | 4 | 6 | 5 | 7 |
| Hospital pharmacies and pharmaceutical centres | 18 | 18 | 14 | 13 |
| Pharmacies and subsidiary pharmacies | 28 | 36 | 55 | 55 |
| Pharmaceutical wholesalers | 25 | 27 | 26 | 29 |
| Tissue establishments | 25 | 25 | 22 | 22 |
| Organ donation and transplantation services | 0 | 6 | 6 | 6 |
| Inspections concerning manufacturers of medical devices | 9 | 9 | 19 | 19 |
| Inspection of authorised representatives, importers and distributors of medical devices | 2 | 3 | 4 | 11 |
| Inspection of the site of f clinical trials of medical devices | 0 | 2 | 1 | 2 |
| Annual monitoring of the notified body | 3 | 3 | 3 | 5 |
| Inspection of a sterilisation service provider | 0 | 0 | 0 | 0 |
In 2024, Fimea carried out more inspections of the pharmaceutical industry than in previous years. The increase in the number of inspections reflects both the introduction of new medicines on the market and the significant construction and expansion projects of the domestic pharmaceutical industry. In 2024, Fimea’s scope of supervision extended to cover seven new pharmaceutical factories outside the EU, and six domestic pharmaceutical factory inspections were related to major construction projects or applying for new factory permits. The factories inspected by Fimea manufacture both sterile medicines (e.g. cancer medicines, insulin, psychiatric medicines, contraceptives, gene therapy medicines and vaccines) and non-sterile medicines (e.g. tablets, inhalers and plasters). Fimea also inspects manufacturers of active substances outside the EU as part of the EDQM inspection programme, which EU medicines agencies do not otherwise routinely inspect.
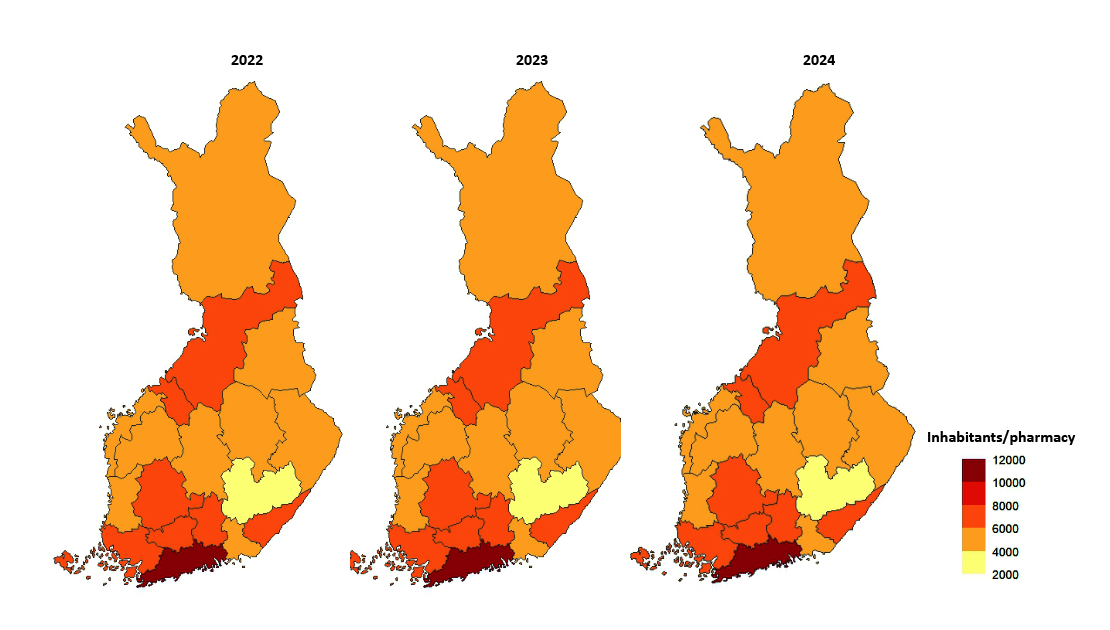
The number of pharmacies (main and secondary pharmacies) in proportion to the number of inhabitants in 2022-2024.
- A total of 648 pharmacies and 185 subsidiary pharmacies were operating in Finland on 31 December 2024. Nine new pharmacies started operations.
- Fimea collects information on the population’s experiences of the availability of medicines at regular intervals to ensure, among other things, the coverage of the Finnish pharmacy network. More than 2,000 adult Finns responded to the Medicines Barometer population survey (Julkari, in Finnish). Of the respondents, 92 per cent felt that pharmacies were close enough to provide easy access to medicines. Only few felt that medicines were not readily available on account of the scarcity of outlets retailing them. Still, one quarter of the respondents (26%) had often been in situations where they needed medicines while the pharmacy was closed.
- Fimea supervises the mandatory reserve supplies of medicines, which safeguards the availability of medicines in Finland during disruptions. If necessary, parties subject to this requirement can be granted a permit to fall below the mandatory reserve supply to ensure the continuous availability of medicines. The realisation of the mandatory reserve supplies of medicines is supervised with inspections.
In 2024, Fimea engaged in national work to promote the security of supply of medicines by participating in working groups, through dialogue with pharmaceutical industry operators and in accordance with separate assignments of the Ministry of Social Affairs and Health. - Fimea is involved in national preparedness activities. Fimea participated in the activities of the Advisory Board for Health and Welfare in Emergency Conditions in matters related to both medicines and medical devices. The Advisory Board was appointed by the Ministry of Social Affairs and Health to promote preparedness for emergency conditions in social welfare and health care and environmental health care. In addition to the Advisory Board, Fimea participated in pool and sector activities coordinated by the National Emergency Supply Agency whose aim was to develop the security of supply and continuity management in the network of companies and organisations. In 2024, Fimea participated in a working group of the National Emergency Supply Agency that aimed to prepare an independent production guide for medicinal oxygen.
Improving the security of supply of blood products has been addressed with supervision measures. Preparedness procedures have been discussed with the ministry steering the operator. Preparedness planning for blood products was also launched in 2024.
National Preparedness Day
The National Preparedness Day takes place annually on 7 February. We participated in the Preparedness Day alongside other authorities. We used our website and social media channels to remind citizens that the 72-hour preparedness supply kits at home should also include the necessary medicines. People should remember to renew their prescriptions in good time, and everyone’s home supplies should cover medicines and first aid supplies for a few days. Additional considerations include a backup power supply for any critical and power-intensive medical devices in use, and spare batteries are necessary for health monitoring devices, such as blood pressure monitors.